-
NewsNews
-
Filter technology
Filter technology
-
Hose systems
Hose systems
-
Publications
Publications
-
Trade fairs
Trade fairs
-
Filter technology
-
Textile filtrationTextile filtration
-
Filter cloths
-
Filter bags
Filter bags
-
Filter belts
Filter belts
-
More products
More products
-
Service filter technology
Service filter technology
-
Areas of application filter technology
Areas of application filter technology
-
Filter cloths
-
Hoses technologyHoses technology
-
Hoses
-
Couplings & connections
Couplings & connections
-
Accessories
Accessories
-
Service hoses technology
Service hoses technology
-
Areas of application hoses technology
Areas of application hoses technology
-
White Paper
White Paper
-
Hoses
-
Other applications
Other applications
-
About usAbout us
-
Markert Group
Markert Group
-
Kunstforum Markert
Kunstforum Markert
-
Management & advisory board
Management & advisory board
-
Research and development
Research and development
-
Quality and the environment
Quality and the environment
-
Production system
Production system
-
Markert Group
-
CareersCareers
-
Training
Training
-
Jobs
Jobs
-
Work at Markert
Work at Markert
-
Training
-
Contact
Contact
Single-use hose systems
Using single use hose systems in pharmaceutical and biotechnology applications
1. Definition Single-Use-Systeme
The term ‘single-use systems’, or ‘disposal systems’ (systems that can be thrown away), describes systems that are only intended to be used one time and then replaced.
In contrast to this, components made of stainless steel or glass can be reused after they have been properly cleaned.
Single-use systems have the advantage over these reusable systems that the initial investment is lower and also energy costs for cleaning processes (CIP, SIP) can be eliminated.
The parts that come in contact with the product consist of materials approved by the Food and Drug Administration (FDA), such as silicone, polyethylene or polycarbonate. Single-use systems are generally manufactured in class 7 cleanrooms and may also be beta or gamma sterilised, which means that they can be used immediately (ready-to-use systems).

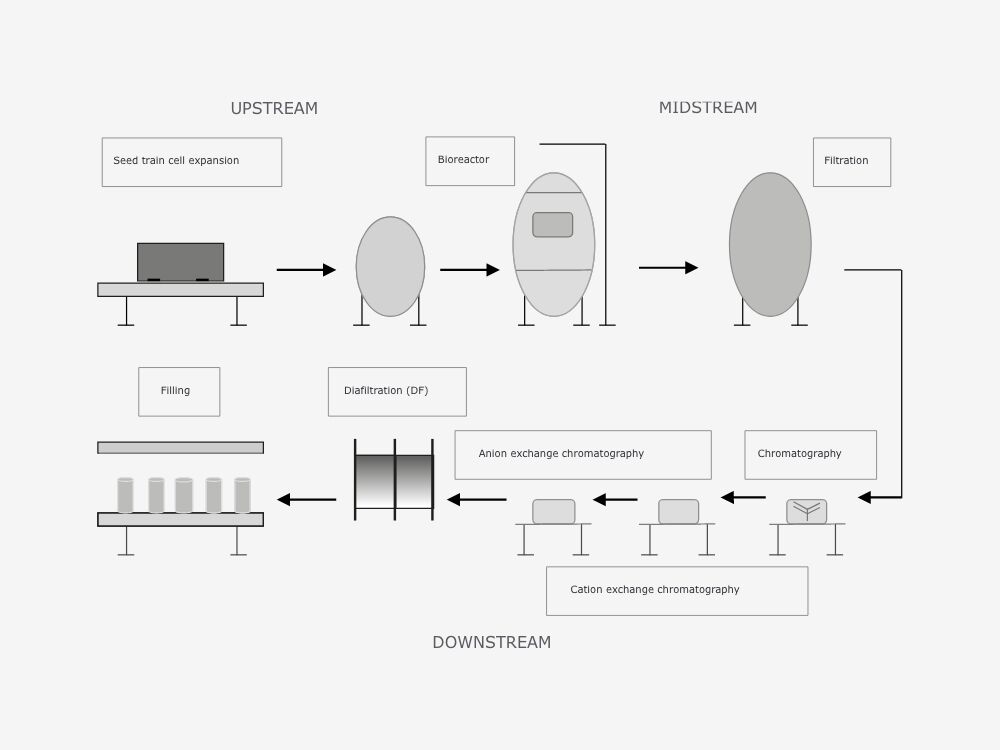
2. Application and processes
The upstream process (USP) includes the initial phase in which the microbes/cells, e.g. bacteria or mammalian cell lines, are bred in bioreactors.
This includes all steps associated with inoculum development: media preparation, cell culture and cell separation, as well as harvest. In biotechnology, ‘inoculum’ is the term for the quantity of reproductive cells with which a fermenter is inoculated. When the cells have reached the desired density, they will be harvested and sent into the bioprocess downstream.
Single-use systems have been used widely in the upstream process for many years (e.g. single-use filters, mixers and bioreactors or single-use plastic bags for storage purposes). The downstream process (DSP) covers the part of a bioprocess in which the cell mass from the upstream is processed in order to meet requirements for purity and quality.
The objective of the downstream process is to isolate and purify a biotechnological product in order to create a form suitable for the intended purpose. The end product in this process is typically recovered from an aqueous solution. End products may include, for example, whole cells, organic acids, amino acids, solvents, antibiotics, industrial enzymes, therapeutic proteins or vaccines.
Due to the variety in size and quality of the products, different classification principles are used in recovery and purification. While single-use systems already see widespread use in the upstream process, they have only recently been increasingly used in the downstream process (e.g. single-use bioreactors for microorganisms).
The final step of the downstream process is formulation (the optimal combination of an active ingredient with certain auxiliary ingredients) and filling. Here, single-use systems are used for mixers, transfer systems, dosing systems and filling needles, among other things.

3. Pros and cons of single-use systems
Single-use systems have the following advantages over reusable equipment made of glass or steel:
- shorter production times (no need for sterilisation and purification procedures)
- higher flexibility (product changes can be performed more quickly)
- higher production security (minimised risk of product cross-contamination)
- lower initial investment in a production plant
Some studies also show that the energy costs for purification (CIP, SIP) of permanent systems are currently higher than those of single-use systems. Thus single-use systems have a better carbon footprint.
On the other hand, they have the following disadvantages:
- Single-use systems (especially in the downstream process) frequently form bottlenecks with higher volumes
- the running costs for consumables are much higher
- potential supply chain issues
- large quantities of waste.


4. Leachables und Extractables
Leachables and extractables (L&E) refer to chemical substances that in the worst-case scenario, migrate out of plastic under process conditions and damage the product. In the extractables study (extraction study), the quantity of organic and inorganic substances that can be extracted from the analysed material by adding extraction solvents is recorded. The objective of the extractable study is to simulate a “worst-case scenario” and use the data obtained to support process developers and toxicologists in validation studies.
The leachables study (leachables = leachable components), records those substances that migrate from the analysed material to the sample under real-life conditions. It may also include the assessment of possible secondary reaction products in a drug. Currently the lack of regulations for standardised tests to prove L&E or corresponding analysis protocols is the biggest weak spot.
Markert had extractable studies performed for the products SIL300PTFE, SIL200 and SIL300.

5. Other approvals (BSE, TSE, GMO, ADI)
The pharmaceutical industry may require manufacturers to confirm that their products are free of BSE/TSE/GMO, or animal components and materials originating from animals or cell cultures:
- BSE (Bovine Spongiform Encephalopathy): the spongiform change that occurs in the brain matter of cattle
- TSE (Transmissible Spongiform Encephalopathy): spongiform brain damage that can be transmitted
- GMO (Genetically Modified Organism): genetically modified organisms
The “ADI-free” certificate verifies that the raw materials used in the production of elastomer do not contain any animal-derived ingredients (ADI).

6. Silicone hoses in single-use systems
Its exceptionally good properties make silicone the material of choice in pharmaceutical applications. The material is relatively soft and can withstand a wide range of temperatures.
Numerous types of silicone are safe to use, meaning they are biocompatible.
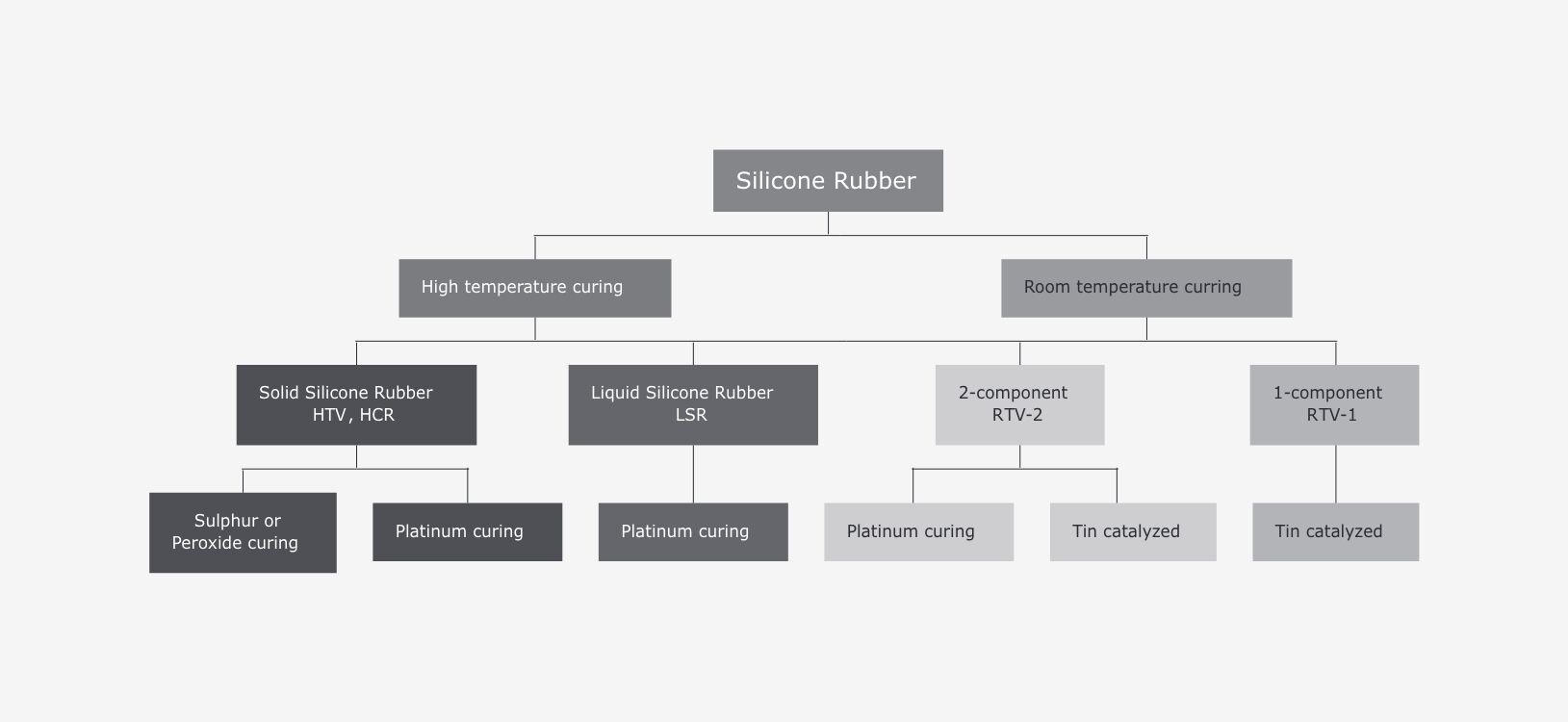
Silicones available on the market today differ in both the pharmaceutical approvals and especially the cross-linking process on which their processing is based.
A distinction is made between platinum-cross-linked and peroxide-cross-linked silicones. Materials that use peroxide to cross-link are exposed to a radical reaction. In doing so, the peroxide group and thus the cross-linking of the silicone is activated. The waste products that form in this process do not dissolve, however, but remain in the end product.
This results in the distinct smell typical of silicone and the ability to leach by-products out of the material.
This is the major risk in using peroxide silicone in pharmaceutical applications.
Platinum-cross-linked silicones have been established very successfully for several years: In these silicones, platinum is used as the catalyst in an addition reaction with silanes. The silane is fully integrated into the silicone and there are no by-products or breakdown products that form.
This means that platinum-cross-linked silicone hoses are completely safe for pharmaceutical applications. It does not take on the unpleasant odour or contain substances that can be leached out in application. The material properties of platinum-cross-linked silicone are comparable to those of materials cross-linked using peroxide.
Silicone hoses used in the pharmaceutical industry are frequently exposed to intense radiation when they are sterilised (gamma radiation or E-Beam). Some studies have shown that gamma or E-Beam radiation has a considerable deteriorating effect on the mechanical properties of peroxide-cross-linked silicone such as hardness, elasticity or ultimate elongation. This deterioration in mechanical properties is not pronounced in platinum-cross-linked silicone.
However, cross-linking notwithstanding, not all silicones are the same. The raw material HCR (abbreviation for High Consistency Rubber, Heat Cured Rubber or HTV, High Temperature Vulcanising) or LSR (Liquid Silicone Rubber) is typically used to manufacture silicone hoses or silicone preforms. HCR is largely cross-linked using peroxide, while LSR is cross-linked with platinum, and has a much lower viscosity. Due to the advantages of platinum-cross-linked silicone described above, LSR is predominantly used as the base material for silicone products in pharmaceutical applications. This is cross-linked through extrusion and made into the desired shape.
LSR (liquid silicone) is offered by various manufacturers and in different qualities. Known and established manufacturers include:
- Wacker
- DuPont
- Dow Momentive Inc
- Shin-Etsu Chemical Co. Ltd. Tokyo
- Elkem in Oslo, Norway
7. Packaging, batch tracking and dimensions
Single-use hoses are typically (depending on the area of application) packaged in cleanrooms in accordance with ISO 14644-1, class 7. The entire process must be set up to allow for the tracking of batches. The systems or components are packed once, but usually twice in PE bags (suitable for gamma sterilisation).
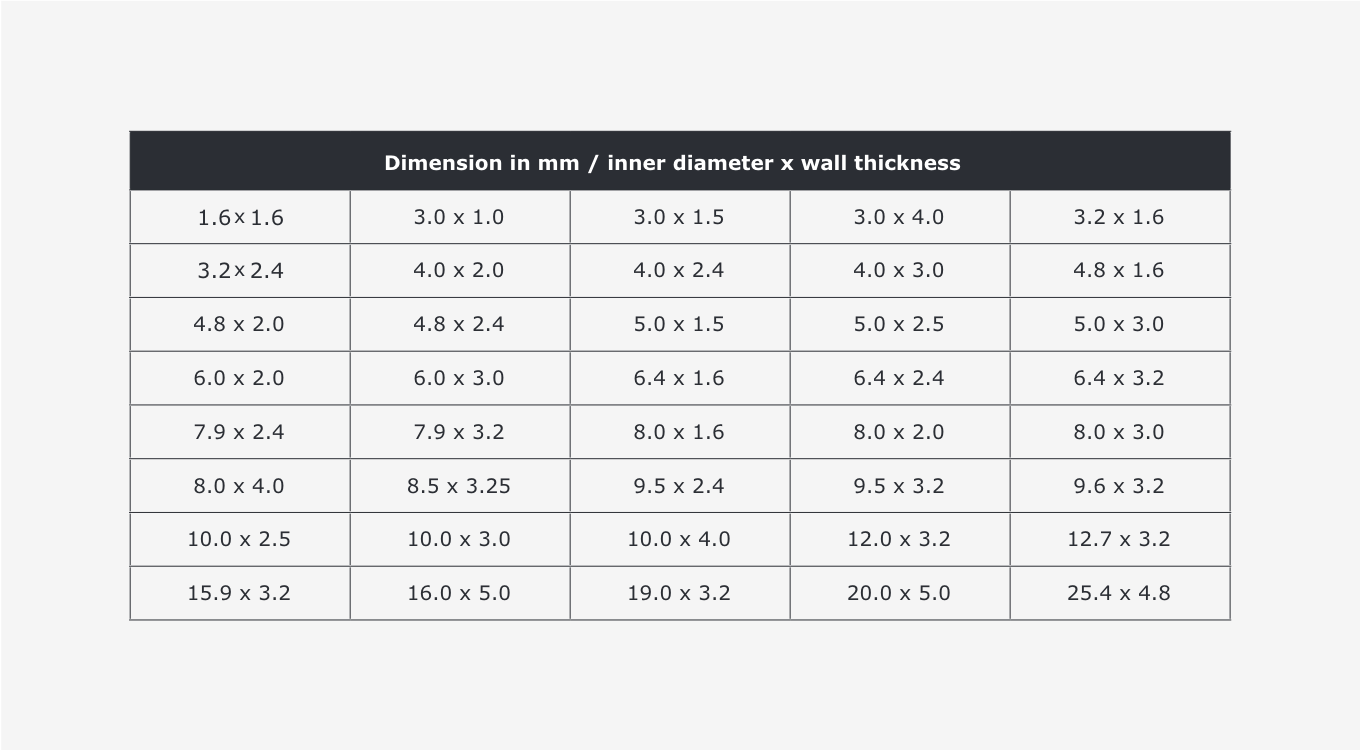
Single-use hoses are available in a wide variety of dimensions. Custom dimensions are generally needed depending on the production process, so there is no uniform standard. The most common dimensions are listed below.
Dimensional tolerances are defined in ISO 3302-1. Three tolerance classes, E1 through E3, are defined here, with E1 representing the lowest tolerances (E1: fine degree of accuracy, E2: moderate degree of accuracy, E3: rough degree of accuracy). Accuracy class E2 generally applies to standardised single-use hoses. Unique products may be manufactured with much lower tolerances (up to a factor of 10)Dimensional tolerances are defined in ISO 3302-1. Three tolerance classes, E1 through E3, are defined here, with E1 representing the lowest tolerances (E1: fine degree of accuracy, E2: moderate degree of accuracy, E3: rough degree of accuracy). Accuracy class E2 generally applies to standardised single-use hoses. Unique products may be manufactured with much lower tolerances (up to a factor of 10).